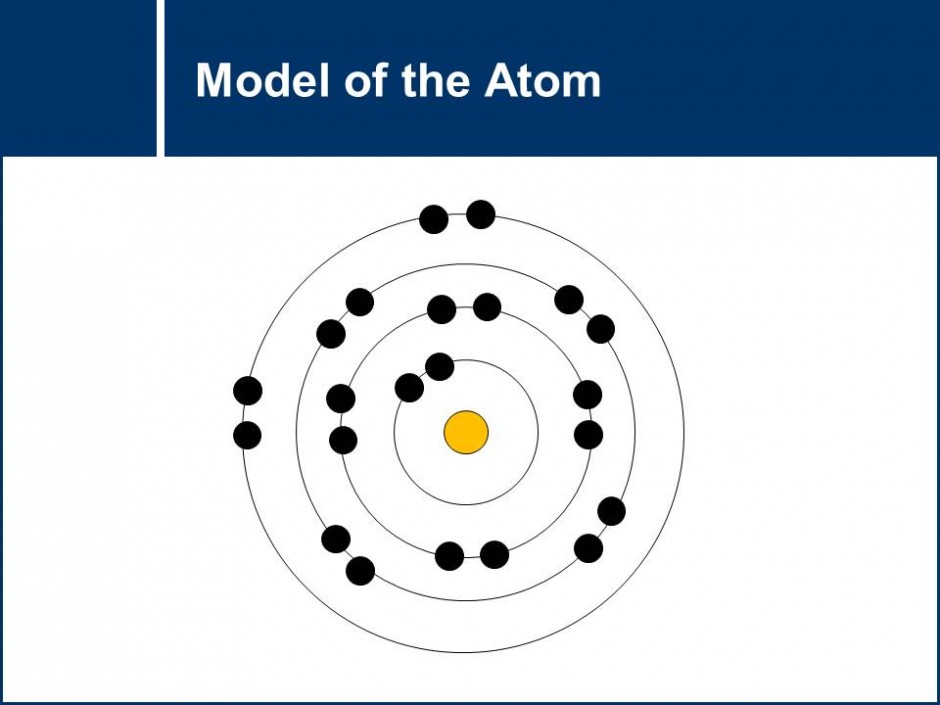
The model of the atom is explored by looking at role and placement of electrons, protons and neutrons in an atom. Questions get students to practice drawing Rutherford-Bohr models of the atom. The role that the gain and loss of electrons have in chemical reactions is also explored which leads to an conclusion about the stability of a full valence electron shell.![]()
![]()
Atomic theory, Bohr, Dalton, electron, electron shell, gaining/losing electrons, model of the atom, neutron, proton, Rutherford, stability of full valence shells, Thomson, Thomson-Rutherford model, valence electrons, valence shell