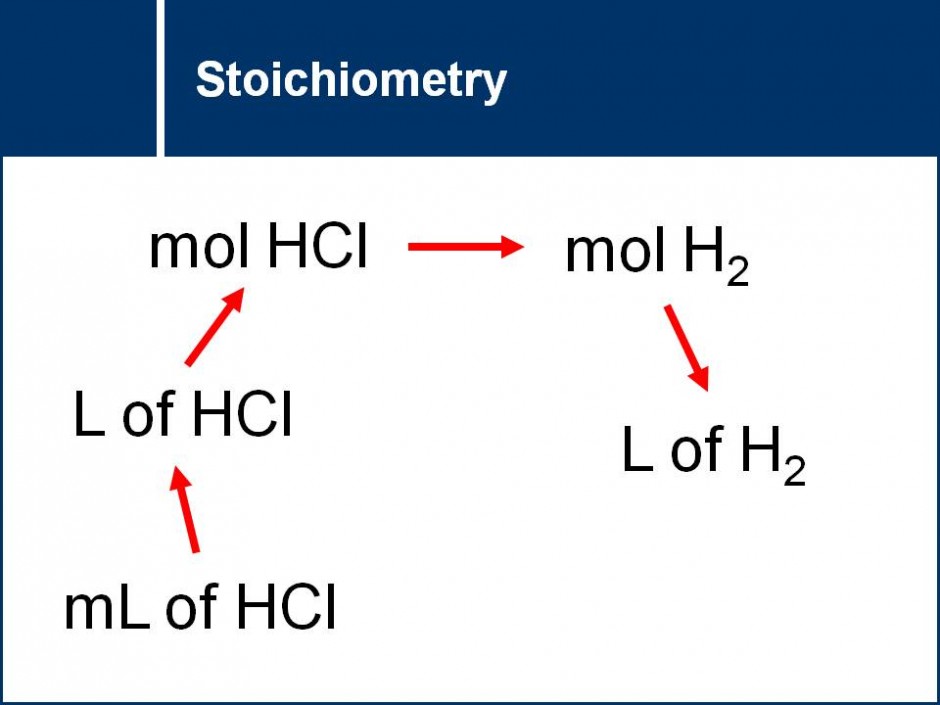
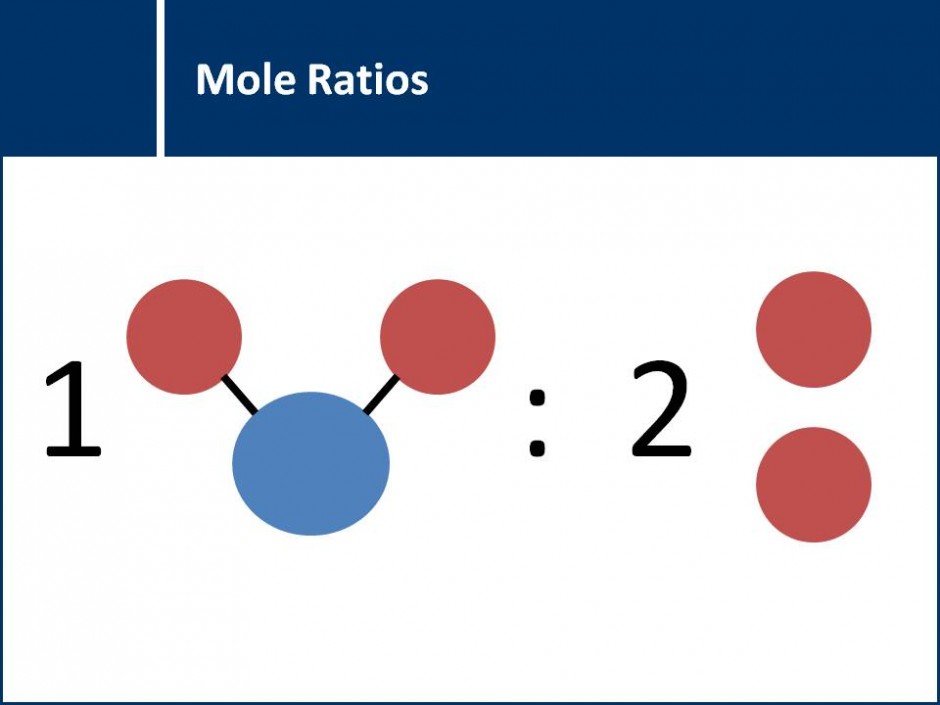
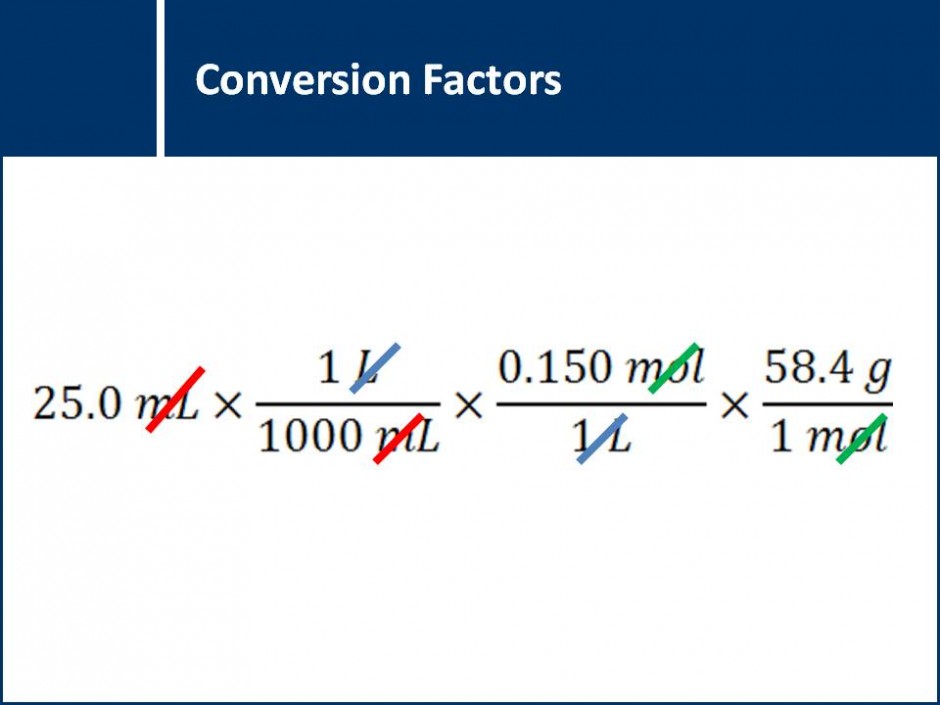
A series of questions that leads students step by step through the process of solving stoichiometry questions. Contains a focus on the best practices for writing out stoichiometry calculations.![]()
![]()
chemistry 11-12, Conversion Factors, molar mass, molar volume of gases, molarity, Mole ratio, stoichiometry
The concept of what the mole is and how the units of atomic mass unit (amu) and grams are related through the Avogadro number are explored. Classification of matter is also discussed regarding the distinction between atoms and molecules.![]()
![]()
atomic mass unit, Avogadro number, chemistry 11-12, classification of matter, mass, molar mass, mole
The concept of mole ratios between the atoms in a molecule and the mole ratios between different compounds in a chemical reaction (and thus balanced chemical equations) are explored. This concept then leads into stoichiometry calculations.![]()
![]()
Balancing chemical equations, chemistry 11-12, classification of matter, Conversion Factors, molar mass, molar volume of gases, mole, Mole ratio, stoichiometry, STP
Different types of mole calculations are explored using the conversion factors of molar mass, concentration, and volume of a gas. An emphasis is placed on correctly setting up the calculations so that units cancel out.![]()
![]()
Avogadro number, chemistry 11-12, Conversion Factors, molar mass, molar volume of gases, molarity, mole, STP